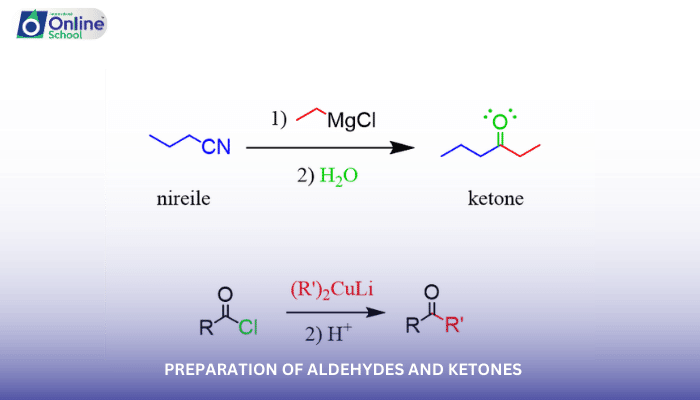
Learning Outcomes:
i. Understand the methods for the preparation of aldehydes and ketones.
ii. Identify the key reactions involved in ozonolysis of alkenes, hydration of alkynes, oxidation of alcohols, and Friedel-Crafts acylation of aromatics.
iii. Relate the preparation methods to the molecular structures of aldehydes and ketones.
Introduction:
Building upon the foundation of nomenclature, this lesson delves into the diverse methods employed in the preparation of aldehydes and ketones. Students will explore the chemical transformations that lead to the formation of these important organic compounds.
i. Ozonolysis of Alkenes:
Ozonolysis is a powerful method for breaking carbon-carbon double bonds in alkenes. Ozone reacts with the alkene, resulting in the formation of aldehydes or ketones depending on the substitution pattern of the alkene. For instance, the ozonolysis of ethene yields formaldehyde, while the ozonolysis of propene can produce acetone.
ii. Hydration of Alkynes:
Alkynes, with their triple bond, can be hydrated to form carbonyl compounds. In the presence of water and a catalyst, the triple bond is broken, and water adds to the carbon atoms. This process is a vital step in the synthesis of ketones. An example is the hydration of acetylene to form acetone.
iii. Oxidation of Alcohols:
Alcohols can be oxidized to produce aldehydes or ketones, depending on the degree of oxidation. Primary alcohols are oxidized to aldehydes, while secondary alcohols are oxidized to ketones. For instance, the oxidation of ethanol results in acetaldehyde, and the oxidation of isopropanol yields acetone.
iv. Friedel-Crafts Acylation of Aromatics:
This method involves the acylation of aromatic compounds using acyl halides or anhydrides in the presence of a Lewis acid catalyst. The reaction leads to the formation of aromatic ketones. For example, the Friedel-Crafts acylation of benzene with acetyl chloride produces acetophenone.
In conclusion, the preparation of aldehydes and ketones involves a variety of synthetic methods, each with its own set of conditions and substrate requirements. From ozonolysis and hydration to oxidation and Friedel-Crafts acylation, these processes contribute to the diverse toolbox of organic chemists, enabling the controlled synthesis of these essential functional groups. Understanding these methods enhances students' grasp of the intricacies of organic chemistry, paving the way for more advanced studies in the field.